IcyEye® Corneal Cryopreservation Kit
Seeking FDA Clearance
Corneas are the most popularly transplanted tissues. Currently, over 10 million patients in the world can benefit from a corneal transplantation surgery. The current and traditional 10 to 14-day hypothermal storage method maintains the qualified viability (over 90%) of the endothelial cell layer, and is vitally important for use in a majority of keratoplasty surgeries; however, the short time period imposes a severe challenge for international transportation and other domestic operations (e.g., in-depth tissue inspections for viruses). In addition, the short time period increases operational costs for eye banks to a considerable degree. Eye banks and the cornea transplantation market should benefit tremendously from introduction of a long-term storage method for use with donor corneas.
Traditional corneal cryopreservation methods have resulted in relatively low post-thaw viability of endothelial cells (typically lower than 70%) and tissue structural damages, and in addition require liquid nitrogen storage facilities (-120°C to -196°C). As a result, no eye banks currently utilize cryopreservation protocols for donor corneas.
The IcyEye® kit comprises a disposable ice-filtering device and C80EZ®-CORNEA, an efficient corneal cryopreservation medium. The device features a nanostructured membrane that filters cell-damaging ice crystals and prevents them from forming in front of the endothelial layer during freezing (international patent filing). The C80EZ®-CORNEA medium enables long-term storage in regular -80°C freezers, as well as safe shipping in dry ice boxes. The use of the IcyEye® kit achieves over 95% post-thaw viability of human corneal endothelial cells, and maintains tissue clarity and normal structures (see Applications). These features allow storage of donor corneas with transplant qualities that are comparable with fresh tissues, even after prominently extended storage of the corneas in regular -80°C freezers.
The C80EZ®-CORNEA medium is chemically compatible with currently used hypothermal storage media (e.g., Optisol and life 4°C). Therefore, the use of the IcyEye® kit can be conveniently adapted into eye bank ongoing operations without requiring any changes in the routine work of the medical professionals doing extraction or for surgeons during transplantation. As such, the eye bank operators can use Optisol or life 4°C medium to transport from donation site to eye banks, then use the IcyEye® kit to cryopreserve corneas in -80°C freezers. The preserved donated corneas can be thawed on an as-needed basis, and transported to specific surgery sites in Optisol or life 4°C medium.
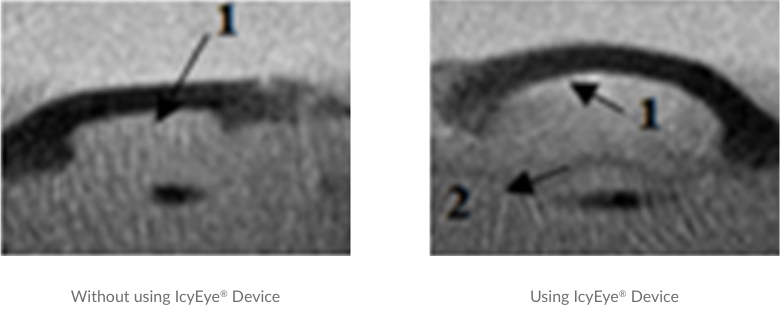
A comparison between the morphology of the growing ice crystals in front of human cornea tissues (~ 2 cm diameter) frozen without (left) and with (right) using IcyEye® based on the observation through a standard nuclear magnetic resonance (NMR) system: the growing ice front “1” approaching the corneal endothelium layer; and the nanostructured membrane “2”. The ice in both cases grows vertically from the bottom to the top of the photo. The ice front near the endothelial layer without the protection of the nanostructured membrane forms sharp edges (shown as black streaks in the figure) that damage cells, while the IcyEye® device filters the ice and removes all damaging ice edges. The ice morphology is different beneath and above “2”, the projection of the membrane in the NMR photography: numerous streaks are seen below “2”, and after being filtered by “2”, the black streaks disappeared and no longer visible, a factor that indicates the ice front is now much smoother.
